Dimorphic fungi: Difference between revisions
From IDWiki
No edit summary |
mNo edit summary |
||
| (4 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | *Fungi that exist in a mold form at lower temperatures in the environment, and a yeast form at higher temperatures in the host body |
||
| − | *[[Cryptococcus]] does exhibit dimorphism, though it is predominately yeast and its dimorphism is not likely related to disease |
||
| − | |||
{| class="wikitable" |
{| class="wikitable" |
||
!Organism |
!Organism |
||
| Line 8: | Line 5: | ||
!Treatment |
!Treatment |
||
|- |
|- |
||
| − | |[[Blastomyces |
+ | |[[Blastomyces]] |
|eastern US and Canada, with some reported in Africa |
|eastern US and Canada, with some reported in Africa |
||
|pulmonary infection, verrucous skin lesions, osteomyelitis, CNS infection |
|pulmonary infection, verrucous skin lesions, osteomyelitis, CNS infection |
||
|[[itraconazole]] (with [[amphotericin B]] induction if severe) |
|[[itraconazole]] (with [[amphotericin B]] induction if severe) |
||
|- |
|- |
||
| − | |[[Coccidioides |
+ | |[[Coccidioides]] |
|southwestern US and parts of South and Central America |
|southwestern US and parts of South and Central America |
||
|pulmonary infection, verrucous skin lesions, osteomyelitis, CNS infection |
|pulmonary infection, verrucous skin lesions, osteomyelitis, CNS infection |
||
|[[fluconazole]] (with [[amphotericin B]] and [[flucytosine]] induction if severe) |
|[[fluconazole]] (with [[amphotericin B]] and [[flucytosine]] induction if severe) |
||
|- |
|- |
||
| − | |[[ |
+ | |[[Histoplasma capsulatum]] |
|worldwide, including eastern North America, Central and South America, sub-Saharan Africa, and parts of Southeast Asia |
|worldwide, including eastern North America, Central and South America, sub-Saharan Africa, and parts of Southeast Asia |
||
|pulmonary infection, CNS infection |
|pulmonary infection, CNS infection |
||
| Line 38: | Line 35: | ||
|[[amphotericin B]] induction followed by [[itraconazole]] |
|[[amphotericin B]] induction followed by [[itraconazole]] |
||
|} |
|} |
||
| − | == |
+ | ==Background== |
| + | ===Microbiology=== |
||
| + | |||
| + | *Broad category of fungi that exist in a mold form at lower temperatures in the environment, and a yeast form at higher temperatures in the host body |
||
| + | *[[Cryptococcus]] does exhibit dimorphism, though it is predominately yeast and its dimorphism is not likely related to disease |
||
| + | *Often referred to as endemic fungi based on their geographic niches |
||
| + | *Includes the following genera and species: |
||
| + | **[[Blastomyces]], including Blastomyces dermatitidis complex ([[Blastomyces dermatitidis]] and [[Blastomyces gilchristii]]), helices, silverae, parvus |
||
| + | **[[Histoplasma capsulatum]] (var. ''capsulatum'' and var. ''duboisii'') |
||
| + | **[[Coccidiodes]], including [[Coccidioides immitis]] and [[Coccidioides posadasii]] |
||
| + | **[[Paracoccidioides]], including [[Paracoccidioides brasiliensis]] and [[Paracoccidioides lutzii]] |
||
| + | **[[Talaromyces marneffei]] |
||
| + | **[[Emergomyces]], including [[Emergomyces pasteurianus]], [[Emergomyces africanus]], [[Emergomyces orientalis]], [[Emergomyces canadensis]], [[Emergomyces europaeus]] |
||
| + | **[[Sporothrix]] complex (Sporothrix brasiliensis, Sporothrix schenckii, Sporothrix globose, Sporothrix luriei) |
||
| + | |||
| + | ===Epidemiology=== |
||
*Endemic dimorphic fungi are widely distributed[[CiteRef::PMID32040709]] |
*Endemic dimorphic fungi are widely distributed[[CiteRef::PMID32040709]] |
||
| − | === |
+ | ====Histoplasmosis==== |
| + | |||
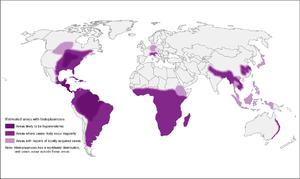
| + | [[File:Histoplasmosis_map.png|Histoplasmosis|alt=|thumb|300x300px]] |
||
| + | |||
| + | *High-endemic areas include Ohio and Mississippi river valley systems, but also in Central and South America |
||
| + | **However, 12-20% of cases in US occur outside of endemic areas |
||
| + | **In Canada, mostly along St. Lawrence seaway and Great Lakes drainage |
||
| + | *More recently, cases have been diagnosed in Alberta and Saskatchewan |
||
| + | *Also found in Asia and Africa, throughout, with var. duboisii in West Africa (mostly skin and soft tissue disease) |
||
| + | *Associated with soil contaminated by bird or bat guano |
||
| + | |||
| + | ====Coccidiomycosis==== |
||
| + | |||
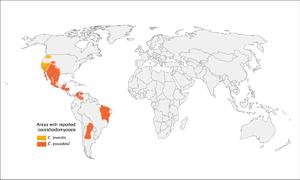
| + | [[File:Coccidiomycosis_map.png|Coccidiomycosis|alt=|thumb|300x300px]] |
||
| + | |||
| + | *More common in southwestern US, especially California and Arizona (but up to Washington state), as well as parts of South and Central America |
||
| + | **Concentrated heavily in San Joaquin Valley in California |
||
| + | *Present in soil |
||
| + | *High-risk activities: construction, military maneuvers, earthquakes/landslides, armadillo hunting, prisoners from other parts of the US that are incarcerated in California |
||
| + | |||
| + | ====Blastomycosis==== |
||
| + | |||
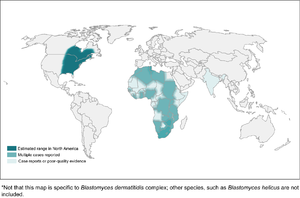
| + | [[File:Blastomycosis_map.png|Blastomycosis|alt=|thumb|300x300px]] |
||
| + | |||
| + | *Found mostly in eastern North America |
||
| + | **In Canada, found in northwestern Ontario, Quebec, Manitoba, and Saskatchewan |
||
| + | ***Kenora is the hotspot in Canada |
||
| + | *More common in wooded areas, damp soil, and near waterways |
||
| + | *High-risk activities include excavation and construction |
||
| + | |||
| + | ====Emergomycosis==== |
||
| + | |||
| + | *Different species found worldwide, including [[Emergomyces canadensis]] in Saskatchewan, Colorado, and New Mexico |
||
| + | *More common in HIV patients or other immunocompromised |
||
| + | |||
| + | ==Clinical Manifestations== |
||
| + | |||
| + | ===Histoplasmosis=== |
||
| + | {| class="wikitable" |
||
| + | ! |
||
| + | !Acute Pulmonary |
||
| + | !Cavitary and Chronic Pulmonary |
||
| + | !Progressive Disseminated |
||
| + | |- |
||
| + | |Clinical |
||
| + | |usually asymptomatic or mild; can have non-pleuritic chest pain from mediastinal or hilar lymphadenopathy; can have rheumatic features or pericarditis |
||
| + | |8% develop fibrocavitary disease, associated with underlying [[COPD]] |
||
| + | | |
||
| + | |- |
||
| + | |Immunology |
||
| + | |>90% positive skin test, 20% urine antigen |
||
| + | |75-95% antibodyes, 40% urine antigen |
||
| + | |60-90% urine antigen |
||
| + | |- |
||
| + | |Culture |
||
| + | |<25% positive |
||
| + | |5-70% positive (more likely if cavitary) |
||
| + | |50-70% positive |
||
| + | |} |
||
| + | |||
| + | ===Blastomycosis=== |
||
| + | |||
| + | *Inhalation is main portal of entry |
||
| + | *Incubation 3 weeks to 3 months |
||
| + | *In outbreaks, 50% of exposed developed symptoms |
||
| + | *Primarily presents with pulmonary blastomycosis with influenza-like illness, acute pneumonia, ARDS, or chronic pneumonia |
||
| + | *Skin is most common extrapulmonary site, but can also infect bone and prostate |
||
| + | *CNS infection is rare |
||
| + | |||
| + | ===Coccidiomycosis=== |
||
| + | |||
| + | *Asymptaomtic common in 60% |
||
| + | *Early pulmonary infection |
||
| + | **Mild |
||
| + | **Valley fever, including arthralgias and erythema nodosum |
||
| + | *Extrapulmonary dissemination |
||
| + | **More common in African Americans |
||
| + | |||
| + | ===Emergomycosis=== |
||
| + | |||
| + | *Cutaneous disease in immunocompromised patients, especially advanced HIV |
||
| + | *Can also cause pulmonary disease, extrapulmonary disease, or disseminated |
||
| + | |||
| + | ==Diagnosis== |
||
| + | |||
| + | *Notify laboratory if a [[Biosafety risk groups|risk group 3]] organism is suspected |
||
| + | *For blood cultures, the isolator system is preferred to BacTAlert |
||
| + | *Media |
||
| + | **Brain-heart infusion with sheep blood plus antibacterials is preferred |
||
| + | **Cycloheximide can be used to prevent growth of saprophytic molds (always with one plate without) |
||
| + | **Incubate at 30ºC to enhance growth of mold forms |
||
| + | **Incubated for 3 weeks for fungi in general, but should be extended to 4 weeks for dimorphic fungi |
||
| + | ***[[Coccidioides]] is the fastest-growing, within 3 to 5 days on SAB, and can grow on chocolate and sheep's blood agars |
||
| + | |||
| + | {| class="wikitable" |
||
| + | !Organism |
||
| + | !Findings on Microscopy |
||
| + | |- |
||
| + | |[[Histoplasma]] |
||
| + | |intracellular 2-4 μm yeast-like cells in macrophages, may have narrow-based budding |
||
| + | |- |
||
| + | |[[Blastomyces]] |
||
| + | |8-15 μm yeast-like cells with broad-based budding, refractile thick cell wall, but early spherules can be confused with [[Coccioides]] |
||
| + | |- |
||
| + | |[[Coccidioides]] |
||
| + | |spherules are thick-walled, 10-80 μm with endospores; alternating barrel-shaped arthroconidia in mycelial form |
||
| + | |- |
||
| + | |[[Marneffei]] |
||
| + | |divides by binary fission |
||
| + | |- |
||
| + | |[[Emergomyces]] |
||
| + | |2.5-5 μm small yeast form with narrow-based budding; septate hyphae with conidiophores at right answers, with conidia clustered in florettes of 2 to 3 conidia |
||
| + | |} |
||
| + | |||
| + | ===EORTC Definition[[CiteRef::donnelly2019re]]=== |
||
| + | *'''Proven invasive fungal disease''' |
||
| − | [[File:Histoplasmosis_map.png|frame|Histoplasmosis]] |
||
| + | **Histopathology or direct microscopy of sterile material of specimens obtained from an affected site showing the distinctive form of the fungus, or |
||
| + | **Recovery by culture of sterile material of the fungus from specimens from an affected site, or |
||
| + | **Blood culture that yields the fungus |
||
| + | *'''Probable invasive fungal disease''' |
||
| + | **Requires clinical features and mycologic evidence, but host does not have to be immunocompromised for dimorphic or endemic fungi |
||
| + | **Host factors: not applicable |
||
| + | **Clinical features: evidence for geographical or occupational exposure (including remote) to the fungus and compatible clinical illness |
||
| + | **Mycological evidence: |
||
| + | ***[[Histoplasma]] or [[Blastomyces]] antigen in urine, serum, or body fluid |
||
| + | ***Antibody to [[Coccidioides]] in CSF or 2-fold rise in 2 consecutive serum samples |
||
| + | ==Prevention== |
||
| − | === Coccidiomycosis === |
||
| + | ===Laboratory Safety=== |
||
| − | [[File:Coccidiomycosis_map.png|frame|Coccidiomycosis]] |
||
| + | *Many are [[Biosafety risk groups|risk group 3]] and need to notify lab if suspected |
||
| − | === Blastomycosis === |
||
| + | *Opening the plates outside of a BSC is one of the highest risk actions |
||
| + | [[Category:Fungi]] |
||
| − | [[File:Blastomycosis_map.png|frame|Blastomycosis]] |
||
Latest revision as of 17:09, 7 March 2024
| Organism | Distribution | Diseases | Treatment |
|---|---|---|---|
| Blastomyces | eastern US and Canada, with some reported in Africa | pulmonary infection, verrucous skin lesions, osteomyelitis, CNS infection | itraconazole (with amphotericin B induction if severe) |
| Coccidioides | southwestern US and parts of South and Central America | pulmonary infection, verrucous skin lesions, osteomyelitis, CNS infection | fluconazole (with amphotericin B and flucytosine induction if severe) |
| Histoplasma capsulatum | worldwide, including eastern North America, Central and South America, sub-Saharan Africa, and parts of Southeast Asia | pulmonary infection, CNS infection | itraconazole (with amphotericin B induction if severe) |
| Paracoccidioides brasiliensis | South America | pulmonary infection | itraconazole (with amphotericin B induction if severe) |
| Sporothrix schenckii | essentially worldwide | nodular lymphangitis | itraconazole |
| Talaromyces marneffei | Southeast Asia | disseminated (common in advanced HIV), pulmonary infection, abdominal abscess, skin lesions, osteomyelitis | amphotericin B induction followed by itraconazole |
Background
Microbiology
- Broad category of fungi that exist in a mold form at lower temperatures in the environment, and a yeast form at higher temperatures in the host body
- Cryptococcus does exhibit dimorphism, though it is predominately yeast and its dimorphism is not likely related to disease
- Often referred to as endemic fungi based on their geographic niches
- Includes the following genera and species:
- Blastomyces, including Blastomyces dermatitidis complex (Blastomyces dermatitidis and Blastomyces gilchristii), helices, silverae, parvus
- Histoplasma capsulatum (var. capsulatum and var. duboisii)
- Coccidiodes, including Coccidioides immitis and Coccidioides posadasii
- Paracoccidioides, including Paracoccidioides brasiliensis and Paracoccidioides lutzii
- Talaromyces marneffei
- Emergomyces, including Emergomyces pasteurianus, Emergomyces africanus, Emergomyces orientalis, Emergomyces canadensis, Emergomyces europaeus
- Sporothrix complex (Sporothrix brasiliensis, Sporothrix schenckii, Sporothrix globose, Sporothrix luriei)
Epidemiology
- Endemic dimorphic fungi are widely distributed1
Histoplasmosis
- High-endemic areas include Ohio and Mississippi river valley systems, but also in Central and South America
- However, 12-20% of cases in US occur outside of endemic areas
- In Canada, mostly along St. Lawrence seaway and Great Lakes drainage
- More recently, cases have been diagnosed in Alberta and Saskatchewan
- Also found in Asia and Africa, throughout, with var. duboisii in West Africa (mostly skin and soft tissue disease)
- Associated with soil contaminated by bird or bat guano
Coccidiomycosis
- More common in southwestern US, especially California and Arizona (but up to Washington state), as well as parts of South and Central America
- Concentrated heavily in San Joaquin Valley in California
- Present in soil
- High-risk activities: construction, military maneuvers, earthquakes/landslides, armadillo hunting, prisoners from other parts of the US that are incarcerated in California
Blastomycosis
- Found mostly in eastern North America
- In Canada, found in northwestern Ontario, Quebec, Manitoba, and Saskatchewan
- Kenora is the hotspot in Canada
- In Canada, found in northwestern Ontario, Quebec, Manitoba, and Saskatchewan
- More common in wooded areas, damp soil, and near waterways
- High-risk activities include excavation and construction
Emergomycosis
- Different species found worldwide, including Emergomyces canadensis in Saskatchewan, Colorado, and New Mexico
- More common in HIV patients or other immunocompromised
Clinical Manifestations
Histoplasmosis
| Acute Pulmonary | Cavitary and Chronic Pulmonary | Progressive Disseminated | |
|---|---|---|---|
| Clinical | usually asymptomatic or mild; can have non-pleuritic chest pain from mediastinal or hilar lymphadenopathy; can have rheumatic features or pericarditis | 8% develop fibrocavitary disease, associated with underlying COPD | |
| Immunology | >90% positive skin test, 20% urine antigen | 75-95% antibodyes, 40% urine antigen | 60-90% urine antigen |
| Culture | <25% positive | 5-70% positive (more likely if cavitary) | 50-70% positive |
Blastomycosis
- Inhalation is main portal of entry
- Incubation 3 weeks to 3 months
- In outbreaks, 50% of exposed developed symptoms
- Primarily presents with pulmonary blastomycosis with influenza-like illness, acute pneumonia, ARDS, or chronic pneumonia
- Skin is most common extrapulmonary site, but can also infect bone and prostate
- CNS infection is rare
Coccidiomycosis
- Asymptaomtic common in 60%
- Early pulmonary infection
- Mild
- Valley fever, including arthralgias and erythema nodosum
- Extrapulmonary dissemination
- More common in African Americans
Emergomycosis
- Cutaneous disease in immunocompromised patients, especially advanced HIV
- Can also cause pulmonary disease, extrapulmonary disease, or disseminated
Diagnosis
- Notify laboratory if a risk group 3 organism is suspected
- For blood cultures, the isolator system is preferred to BacTAlert
- Media
- Brain-heart infusion with sheep blood plus antibacterials is preferred
- Cycloheximide can be used to prevent growth of saprophytic molds (always with one plate without)
- Incubate at 30ºC to enhance growth of mold forms
- Incubated for 3 weeks for fungi in general, but should be extended to 4 weeks for dimorphic fungi
- Coccidioides is the fastest-growing, within 3 to 5 days on SAB, and can grow on chocolate and sheep's blood agars
| Organism | Findings on Microscopy |
|---|---|
| Histoplasma | intracellular 2-4 μm yeast-like cells in macrophages, may have narrow-based budding |
| Blastomyces | 8-15 μm yeast-like cells with broad-based budding, refractile thick cell wall, but early spherules can be confused with Coccioides |
| Coccidioides | spherules are thick-walled, 10-80 μm with endospores; alternating barrel-shaped arthroconidia in mycelial form |
| Marneffei | divides by binary fission |
| Emergomyces | 2.5-5 μm small yeast form with narrow-based budding; septate hyphae with conidiophores at right answers, with conidia clustered in florettes of 2 to 3 conidia |
EORTC Definition2
- Proven invasive fungal disease
- Histopathology or direct microscopy of sterile material of specimens obtained from an affected site showing the distinctive form of the fungus, or
- Recovery by culture of sterile material of the fungus from specimens from an affected site, or
- Blood culture that yields the fungus
- Probable invasive fungal disease
- Requires clinical features and mycologic evidence, but host does not have to be immunocompromised for dimorphic or endemic fungi
- Host factors: not applicable
- Clinical features: evidence for geographical or occupational exposure (including remote) to the fungus and compatible clinical illness
- Mycological evidence:
- Histoplasma or Blastomyces antigen in urine, serum, or body fluid
- Antibody to Coccidioides in CSF or 2-fold rise in 2 consecutive serum samples
Prevention
Laboratory Safety
- Many are risk group 3 and need to notify lab if suspected
- Opening the plates outside of a BSC is one of the highest risk actions
References
- ^ Ashraf N, Kubat RC, Poplin V, Adenis AA, Denning DW, Wright L, McCotter O, Schwartz IS, Jackson BR, Chiller T, Bahr NC. Re-drawing the Maps for Endemic Mycoses.. Mycopathologia. 2020. doi:10.1007/s11046-020-00431-2. PMID 32040709.
- ^ J Peter Donnelly, Sharon C Chen, Carol A Kauffman, William J Steinbach, John W Baddley, Paul E Verweij, Cornelius J Clancy, John R Wingard, Shawn R Lockhart, Andreas H Groll, Tania C Sorrell, Matteo Bassetti, Hamdi Akan, Barbara D Alexander, David Andes, Elie Azoulay, Ralf Bialek, Robert W Bradsher, Stephane Bretagne, Thierry Calandra, Angela M Caliendo, Elio Castagnola, Mario Cruciani, Manuel Cuenca-Estrella, Catherine F Decker, Sujal R Desai, Brian Fisher, Thomas Harrison, Claus Peter Heussel, Henrik E Jensen, Christopher C Kibbler, Dimitrios P Kontoyiannis, Bart-Jan Kullberg, Katrien Lagrou, Frédéric Lamoth, Thomas Lehrnbecher, Jurgen Loeffler, Olivier Lortholary, Johan Maertens, Oscar Marchetti, Kieren A Marr, Henry Masur, Jacques F Meis, C Orla Morrisey, Marcio Nucci, Luis Ostrosky-Zeichner, Livio Pagano, Thomas F Patterson, John R Perfect, Zdenek Racil, Emmanuel Roilides, Marcus Ruhnke, Cornelia Schaefer Prokop, Shmuel Shoham, Monica A Slavin, David A Stevens, George R Thompson, Jose A Vazquez, Claudio Viscoli, Thomas J Walsh, Adilia Warris, L Joseph Wheat, P Lewis White, Theoklis E Zaoutis, Peter G Pappas. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clinical Infectious Diseases. 2019;71(6):1367-1376. doi:10.1093/cid/ciz1008.