Histoplasma capsulatum
From IDWiki
Histoplasma capsulatum
Background
Microbiology
- Saprophytic environmental fungus withing the family Ascomycetes
- Thermally dimorphic, existing as a mold <35ºC and a yeast at >37ºC
- Mold
- Mold form is highly infectious, associated with lab-related outbreaks
- Septate hyaline mold with aerial hyphae with macroconidia, which are its identifying feature
- Two types of conidia: tuberculate macroconidia (ovoid bodies 8 to 15 μm with spikes), and microconidia (small, smooth oval bodies 2 to 5 μm)
- Two colony types, brown (B) and albino (A)
- Yeast
- Non-infectious, once hanging out in your body
- Small, 2 to 5 μm
- Demonstrates multipolar narrow-based budding
- Does not look particularly different from other yeast, but may be intracellular
- Mold
- Three variants
- H. capsulatum var. capsulatum, which is the most common worldwide, and is further divided into various clades
- H. capsulatum var. duboisii which is only present in western Africa, and has larger yeast forms
- Can take up to 7 days to grow
- H. capsulatum var. farciminosum
Epidemiology
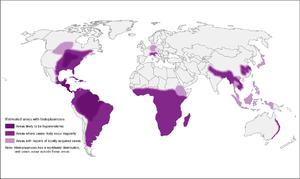
- Endemic in many parts of the world
- Ohio and Mississippi River Valley systems (Central/Eastern US), where seroprevalence is as high as 80% in adults
- Probably up through St. Lawrence River as well
- Probably more broadly distributed, including Central and South America, South and East Asia, and Australia
- H. capsulatum var. duboisii in western Africa
- Typically found in moist soil enriched with bat or bird droppings, which helps it to sporulate
- Disturbing the soil aerosolizes it, allowing the microconidia to be inhaled
- Microconidia can be transported for miles by air currents
Risk Factors
- HIV, solid organ transplant, hematologic transplant
- Primary immunodeficiencies: X-linked hypogammaglobulinemia
Pathophysiology
- Inhaled microconidia reach the alveolii and are phagocytosed by alveolar macrophages
- Innoculum size can be smaller with immunodeficiency
- Size of innoculation affects disease severity and progression
- Microconidia transform into budding yeasts, in a process that is dependent on intracellular macrophage calcium and iron
- They multiply inside macrophages, and translocate through the lymphatics
- Cellular immunity developed around 2 weeks later
- Response depends on IL-12 and TNF-α
- Organize to form granulomas to contain the infection
- Latent infection can reactivate, but rare
- Most common with infliximab
- In impaired cellular immunity, infection can become disseminated
Clinical Manifestations
- Spectrum of illness, related to the size of the inoculum, strain-specific virulence, and host immunity
- Often asymptomatic; in endemic areas, 50-80% of people skin-test positive or have radiographic evidence of previous infection
- Can cross tissue planes
Acute Pulmonary Histoplasmosis
- Fever, chill, malaise, headaches, myalgias, anorexia, cough, dyspnea, and chest pain
- Spectrum from mild to severe
- Usually self-limited, no need to treat unless longer than a month
- Pneumonitis on chest x-ray, often with adenopathy
- "Buckshot" appearance? (Mandell)
- Can have rheumatologic sequelae in 5-10%, with arthralgias, arthritis, and erythema nodosum
- Can have pericarditis from the inflammatory response
- Hilar adenopathy can necrotize
Progressive Disseminated Histoplasmosis
- Usually, though not exclusively, in immunocompromised patients
- Risk factors include CD4 <200, very old or very young, and therapeutic immunosuppression (prednisone, MMF, tacrolimus, methotrexate, TNF-α inhibitors, other biologics)
- Can be rapidly-progressing and acute, or more subacute
Acute Progressive Disseminated Histoplasmosis
- Fever, weight loss, organomegaly, thrombocytopenia
- Meningitis or focal brain lesions
- Oral and GI mucosal ulcerations
- Adrenal insufficiency
Chronic Progressive Disseminated Histoplasmosis
- In normal hosts
- Absent or low-grade fever
- Longer course
- Most common finding is oropharyngeal lesion: deep, well-circumscribed, unrated, and painless
- Mimics squamous cell carcinoma
- Can also have hepatosplenomegaly, chronic meningitis, or chronic granulomatous hepatitis
Chronic Cavitary Histoplasmosis
- Typically seen in bullous emphysema
- Productive cough, dyspnea, low-grade fever, night sweats, weight loss
- Hemoptysis is rare
- Progressive without treatment
- Chest x-ray shows upper-lobe infiltrations, vacitation, and pleural thickening, similar to tuberculosis
Fibrosing Mediastinitis
- Histoplasmosis is the most common cause of fibrosing mediastinitis
- Rare but serious
- Progressive fibrosis around hilar/mediastinal lymphadenopathy, wither unilateral or bilateral
- Occludes central vessels and airways
- Can present with a SVC syndrome, obstruction of pulmonary vessels, or airway obstruction
- Can also present with recurrent pneumonias, hemoptysis, or respiratory failure
- 30% mortality
Other Complications
- Ophthalmic posterior uveitis
- Meningitis
- Infective endocarditis
African Histoplasmosis
- H. capsulatum vars. capsulatum and duboisii coexist in Africa
- var. duboisii has more skin and skeletal manifestations
- Ulcers, nodules, or psoriaform lesions that can spontaneously resolve
- Can cause a cold abscess, without inflammation
- Osteolytic bone lesions are common (50%) of cases
- Skull and ribs most common
- Can have sinus formation and cystic bone lesions
- May not have any evidence on CXR of prior pulmonary histoplasmosis
- Can also present with progressive disseminated disease, with fevers and multiorgan involvement
- Combianation of granulomas and pus
- Larger yeast is harder for macrophages to engulf
- Ulcers, nodules, or psoriaform lesions that can spontaneously resolve
Diagnosis
- Histopathology of biopsy specimens
- Caseating and non-caseating granulomas
- Mold and yeast forms depending on the temperature
- Best stain is GMS (Gomori methenamine silver)
- Seen within the macrophages
- Fungal culture of sputum (chronic cavitary), or blood or bone marrow aspirate (disseminated), or CSF (CNS histo)
- Usually grows within 7 days, and almost always within 21 days
- Need to use lysis centrifugation system to release intracellular pathogens before culture
- Yield of 15% for acute pulmonary, but cavitary is 60% and up to 90% in advanced HIV with bronchoscopy
- Bone marrow and blood cultures are 50% sensitive
- Sensitivity increases with volume and number of samples
- Serology can be done for antigen or antibody
- Serology for antibodies by complement fixation
- Serology for antibodies by agar gel precipitin test
- Anti-H is uncommon (<10% of patients), but signifies active infection
- Anti-M is common (up to 80% of patients), but signifies either active or recovered infection
- Serology may be negative in immunosuppressed patients
- Antigen of urine (best), BAL fluid, and serum if available
- Urine is best, but only 40% sensitive in cavitary, up to 95% in AIDS patients
- However, this may no longer be the case, with overall sensitivity of 80% and specificity of 90% regardless of sample source
- Cross-reacts with other endemic fungi; false-positives with antithymocyte globulin
- Urine is best, but only 40% sensitive in cavitary, up to 95% in AIDS patients
- PCR is possible
- 16S PCR
Management
- In general, mild infections are treated with itraconazole and severe infections with amphotericin B
- Give tablets of itraconazole with acidic drink, such as can of soda, and avoid antacids
- Itraconazole requires therapeutic drug monitoring
- Voriconazole is an easier-to-prescribe alternative that is likely as effective as itraconazole
- Indications for antifungal therapy
- Definitely: moderate to severe acute diffuse pulmonary infection, chronic cavitary pulmonary disease, disseminated disease, CNS infection
- Possibly/uncertain: asymptomatic, mild symptoms lasting longer than 1 month, acute focal pulmonary infection, mediastinal lymphadenitis, mediastinal granuloma
- Not recommended: mediastinal fibrosis, pulmonary nodule, broncholithiasis, presumed ocular histoplasmosis syndrome
| Syndrome | Treatment |
|---|---|
| Acute pulmonary histoplasmosis | |
| Mild, self-resolving | If resolves within a month, no need to treat |
| Mild, ongoing symptoms | Itraconazole 200 mg po TID x3d then itra 200 mg po daily or BID for 6-12 weeks |
| Moderate to severe | Liposomal amphotericin B 3-5 mg/kg/d for 1-2 weeks, followed by itraconazole 200 mg TID x3d then itraconazole 200 mg BID x12wk Methylprednisolone 0.5-1 mg/kg IV daily for first 1-2 weeks if respiratory complications |
| Chronic cavitary pulmonary histoplasmosis | Itraconazole 200 mg TID x3d then daily or BID for at least 1 year (18-24 months may have lower relapse) |
| Complications | |
| Pericarditis | NSAIDs if mild Prednisone 0.5-1 mg/kg daily then taper over 1-2 weeks, plus itraconazole (as above) for 6-12 weeks if hemodynamic compromise May need therapeutic pericardiocentesis |
| Rheumatologic | NSAIDs if mild, prednisone and itraconazole (as for pericarditis) if severe |
| Mediastinal lymphadenitis | Usually no treatment. Follow guide for acute pulmonary histoplasmosis. |
| Mediastinal granuloma | Usually no treatment. Standard itraconazole protocol for 6-12 weeks if symptomatic. |
| Mediastinal fibrosis | Antifungals not recommended. Treat only if there is suspicion of mediastinal granuloma. May need stenting of obstructed pulmonary vessels. |
| Broncholithiasis | Antifungals not recommended. May need surgery. |
| Progressive disseminated histoplasmosis | Follow antigen levels during therapy and for 12 months after to monitor for relapse |
| Mild to moderate | Itraconazole for 12 months |
| Moderately severe to severe | Liposomal amphotericin B 3 mg/kg for 1-2 weeks then oral itraconazole for at least 12 months |
| Immunosuppressed | May need lifelong suppressive therapy with itraconazole 200 mg po daily |
| CNS histoplasmosis | Liposomal amphotericin B 5 mg/kg daily for 4-6 weeks (total 175 mg/kg) followed by itraconazole for at least 1 year, until resolution of CSF abnormalities |
| Pregnancy | Liposomal amphotericin B 3-5 mg/kg for 4-6 weeks |
| Children | As per above guidelines, with amphotericin B deoxycholate 1 mg/kg and itraconazole 2.5-5 mg/kg bid (max 400 mg daily) |
| Prophylaxis | Itraconazole 200 mg po daily recommended if HIV with CD4 <150 and more than 10 cases per 100 patient-years |
- Note: therapeutic drug level monitoring is recommended for itraconazole
- Source: IDSA guidelines 2007
Prevention
Lab Safety
- Biosafety risk group 3 organism, so needs BSL 3
- Should be suspected with any white mold
Prophylaxis
- May be indicated for endemic areas in patients with advanced HIV and low CD4 count