Helicobacter pylori
From IDWiki
Helicobacter pylori
Background
- Slow-growing Gram-negative microaerophilic bacillus with a curve, gull-wing, or spiral appearance
- Oxidase-positive and urease-positive
- Major cause of peptic ulcer disease and gastric cancer worldwide
Pathophysiology
- Urease neutrolizes acid and induces angiogenesis
- Strains with CagA, VacA, and BabA are associated with more cellular metaplasia
Epidemiology
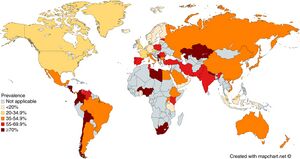
- Present worldwide
- About half of the world's population is estimated to have chronic infection[1]
- Usually acquired during infancy or childhood
- Transmission is likely fecal-oral or oral-oral
Management
- Treatment is with combination therapy for 14 days followed by confirmation of eradication
- First-line:
- PBMT (PPI, bismuth, metronidazole, tetracycline) (BMT Quad)
- PAMC (PPI, amoxicillin, metronidazole, clarithromycin) (CLAMET Quad)
- PAC (PPI, amoxicillin, clarithromycin), PMC (PPI, metronidazole, clarithromycin), or PAM (PPI, amoxicillin, metronidazole) only in areas with clarithromycin resistance <15% or with proven high local eradication rates >85%
- Prior treatment failure:
- PBMT (PPI, bismuth, metronidazole, tetracycline)
- PAL (PPI, amoxicillin, levofloxacin)
- PAR (PPI, amoxicillin, rifabutin) for 10 days, as last-line
- Doses:
- PBMT
- Bismuth subsalicylate 524 mg (2x 262 mg tablets) PO qid
- Metronidazole 500 MG PO tid or qid
- PPI: esomeprazole 20 mg, lansoprazole 30 mg, omeprazole 20 mg, pantoprazole 40 mg, or rabeprazole 20 mg
- Some areas use double dosing
- Tetracycline 500 mg PO qid
- Others
- Amoxicillin 1000 mg PO bid
- Clarithromycin 500 mg PO bid
- Levofloxacin 500 mg PO daily
- Metronidazole 500 mg PO bid
- Rifabutin 150 mg PO bid
- PPI as above
- PBMT
- Duration: 14 days
- Confirmation of eradication should be done 4 weeks following treatment
- Recommended order of treatment, if persistently positive:
- PBMT (or PAMC)
- PAMC (or PBMT)
- PAL
- PAR vs. repeat endoscopy for culture and susceptibility testing
Further Reading
- H. pylori Enhanced Primary Care Pathway: 2016 version, 2019 version, 2020 version
- The Toronto Consensus for the Treatment of Helicobacter pylori Infection in Adults. Gastroenterol. 2016;151:51–69. doi: 10.1053/j.gastro.2016.04.006
- Houston Consensus Conference on Testing for Helicobacter pylori Infection in the United States. Clin Gastroenterol Hepatol. 2018;16(7):992-1002.e6. doi: 10.1016/j.cgh.2018.03.013
- ↑ Zamani M, Ebrahimtabar F, Zamani V, Miller WH, Alizadeh-Navaei R, Shokri-Shirvani J, Derakhshan MH. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. 2018 Apr;47(7):868-876. doi: 10.1111/apt.14561. Epub 2018 Feb 12. PMID: 29430669.
References
- ^ M. Zamani, F. Ebrahimtabar, V. Zamani, W. H. Miller, R. Alizadeh‐Navaei, J. Shokri‐Shirvani, M. H. Derakhshan. Systematic review with meta‐analysis: the worldwide prevalence of Helicobacter pylori infection. Alimentary Pharmacology & Therapeutics. 2018;47(7):868-876. doi:10.1111/apt.14561.
- ^ G. Manes, A. Balzano, G. Iaquinto, C. Ricci, M. M. Piccirillo, N. Giardullo, A. Todisco, M. Lioniello, D. Vaira. Accuracy of the stool antigen test in the diagnosis of Helicobacter pylori infection before treatment and in patients on omeprazole therapy. Alimentary Pharmacology & Therapeutics. 2001;15(1):73-79. doi:10.1046/j.1365-2036.2001.00907.x.