Echinococcus granulosus: Difference between revisions
From IDWiki
Echinococcus granulosus
Content deleted Content added
added table of ultrasound features |
|||
| Line 68: | Line 68: | ||
**Transitional disease (CE3): cysts are starting to degenerate but the protoscolices are still viable |
**Transitional disease (CE3): cysts are starting to degenerate but the protoscolices are still viable |
||
**Inactive disease (CE4-CE5): cysts are fully degenerated and are now calcifying |
**Inactive disease (CE4-CE5): cysts are fully degenerated and are now calcifying |
||
{| class="wikitable" |
|||
!Type |
|||
!Imaging |
|||
!Status |
|||
!Description |
|||
|- |
|||
|CL |
|||
|Unilocular, cystic lesion with uniform anechoic content, no clearly delimited by a hyperechoic rim (cyst wall not visible). Normally round byt may be oval. |
|||
|Active |
|||
|Usually non-parasitic cystic lesions, but can be seen in early disease and are not fertile. |
|||
|- |
|||
|CE1 |
|||
|Unilocular, simple cyst with uniform anechoic content. Cyst may exhibit fine echoes due to shifting of brood capsules which is often called hydatid sand ("snow flake sign"). Cyst wall is visible. Normally round or oval. |
|||
|Active |
|||
|Usually fertile. Pathognomonic signs are visible cyst wall and snowflake sign. |
|||
|- |
|||
|CE2 |
|||
|Multivesicular, multiseptated cysts in which the daugher cysts may partly or completely fill the unilocular mother cyst. Cyst septations may produce "wheel-like" structures, or the daughter cysts may produce a "rosette-like" or "honeycomb-like" structure. Normally round or oval. |
|||
|Active |
|||
|Usually fertile. |
|||
|- |
|||
|CE3 |
|||
|Anechoic content with detachment of laminated membrane from the cyst wall visible as floating membrane of as "water-lily sign", which is indicative of wavy membranes floating on top of remaining cyst fluid. Unilocular cyst which may contain daughter cysts (anechoic appearance) and echoic areas (disrupted memranes/degenerating daughter cysts). Cyst may be less rounded. |
|||
|Transitional |
|||
|Cyst usually starting to degenerate. |
|||
|- |
|||
|CE4 |
|||
|Heterogeneous hypoechoic or dyshomogeneous degenerative contents. No daughter cysts. May show a "ball of wool sign", which is indicative of degenerating membranes. |
|||
|Inactive |
|||
|Most cysts are not fertile. |
|||
Ultrasound features are not pathognomonic. |
|||
|- |
|||
|CE5 |
|||
|Cysts characterised by thick calcified wall which is arch shaped, producing a cone shaped shadow. Degree of calcification varies from partial to complete. |
|||
|Inactive |
|||
|Majority of cysts not fertile. |
|||
Ultrasound features are not pathognomonic. |
|||
|} |
|||
*Pathognomonic features include: |
*Pathognomonic features include: |
||
**Unilocular anechoic lesion that are round or oval with a clear, well-defined wall, and souble line sign |
**Unilocular anechoic lesion that are round or oval with a clear, well-defined wall, and souble line sign |
||
Revision as of 03:59, 14 July 2023
Background
Microbiology
- Cestode in the Echinococcus family
- Multiple strains cause hydatid cysts
- Strains have host specificity and are organised by genotype (G1 to G10):
- Echinococcus granulosus sensu stricto (G1 to G3), the most common cause worldwide
- Echinococcus equinus (G4), with no human cases reported
- Echinococcus ortleppi (G5)
- Echinococcus canadensis (G6 to G10)
Life Cycle
- Carnivores are the definitive host (canines, felids, or hyenids), where the protoscolex develops into a cestode (after 4 to 7 weeks) and releases eggs
- Eggs are infectious on release, and can be viable in the environment for months or longer
- Intermediate hosts are usually herbivores (sheep, goats, cattle, camels, and cervids), where the oncosphere hatches, penetrates the intestine, and migrates to the target organ, where it encysts to create the metacestode (or hydatid cyst)
- Within the cyst, protoscolices develop to form a germinal layer
- The protoscolices are ingested by the definitive host
Epidemiology
- Present in South America, the Middle East and eastern Mediterranean, some sub-Saharan African countries, western China, and eastern Europe
- Exposure to sheep or other livestock, especially when dogs are kept and fed offal
Clinical Manifestations
- Larval form can affect any organ, and most commonly (40-80%) as a single lesion
- Most common single sites are the liver (80%) and lungs (20%)
- E. canadensis may be more likely to go to brain
- Other sites include pleural cavity, kidney, spleen, bone, brain, eye, ovary, testis, and pancreas
- Cysts grow by 1 to 50 mm annually, and can spontaneously rupture
- Symptoms are caused by compression of nearby structures or by rupture
- Cysto-biliary fistula is the most common complication of liver cysts
Rupture
- Cyst rupture releases protoscolices into nearby tissues
- This causes an anaphylactic reaction
Diagnosis
- Based on a combination of clinical picture, imaging, and serology
Case Definition
- Clinical criteria must include one of:
- Slowly-growing or static cystic mass(es) (signs and symptoms vary with cyst location, size, type, and number) diagnosed by imaging technique
- Anaphylactic reactions due to ruptured or leaking cysts
- Incidental finding of a cyst by imaging techniques in asymptomatic carriers or detected by screening strategies
- Diagnostic criteria
- Typical organ lesion(s) detected by imaging technique (e.g. US, CT scan, plain film radiography, MR imaging)
- Specific serum antibodies assessed by high-sensitivity serological tests, confirmed by a separate high specificity serological test
- Histopathology or parasitology compatible with cystic echinococcosis (e.g. direct visualization of the protoscolex or hooklets in cyst fluid)
- Detection of pathognomonic macroscopic morphology of cyst(s) in surgical specimens
- Interpretation
- Possible case: any patient with a clinical or epidemiological history, and imaging findings or serology positive
- Probable case: any patient the the combination of clinical history, epidemiological history, imaging findings, and serology positive on two tests
- Confirmed case: the above, plus either demonstration of protoscoleces or their components, using direct microscopy or molecular biology, in the cyst contents aspirated by percutaneous puncture or at surgery, or changes in US appearance (e.g. detachment of the endocyst in a CE1 cyst, thus moving to a CE3a stage, or solidification of a CE2 or CE3b, thus changing to a CE4 stage) after administration of albendazole for at least 3 months or spontaneous.
Ultrasound
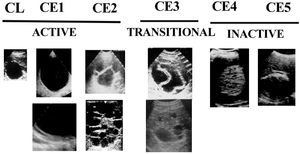
- Standardized ultrasound classifications have been created by the WHO1
- Active disease (CE1-CE2): cysts are developing and are usually fertile
- Transitional disease (CE3): cysts are starting to degenerate but the protoscolices are still viable
- Inactive disease (CE4-CE5): cysts are fully degenerated and are now calcifying
| Type | Imaging | Status | Description |
|---|---|---|---|
| CL | Unilocular, cystic lesion with uniform anechoic content, no clearly delimited by a hyperechoic rim (cyst wall not visible). Normally round byt may be oval. | Active | Usually non-parasitic cystic lesions, but can be seen in early disease and are not fertile. |
| CE1 | Unilocular, simple cyst with uniform anechoic content. Cyst may exhibit fine echoes due to shifting of brood capsules which is often called hydatid sand ("snow flake sign"). Cyst wall is visible. Normally round or oval. | Active | Usually fertile. Pathognomonic signs are visible cyst wall and snowflake sign. |
| CE2 | Multivesicular, multiseptated cysts in which the daugher cysts may partly or completely fill the unilocular mother cyst. Cyst septations may produce "wheel-like" structures, or the daughter cysts may produce a "rosette-like" or "honeycomb-like" structure. Normally round or oval. | Active | Usually fertile. |
| CE3 | Anechoic content with detachment of laminated membrane from the cyst wall visible as floating membrane of as "water-lily sign", which is indicative of wavy membranes floating on top of remaining cyst fluid. Unilocular cyst which may contain daughter cysts (anechoic appearance) and echoic areas (disrupted memranes/degenerating daughter cysts). Cyst may be less rounded. | Transitional | Cyst usually starting to degenerate. |
| CE4 | Heterogeneous hypoechoic or dyshomogeneous degenerative contents. No daughter cysts. May show a "ball of wool sign", which is indicative of degenerating membranes. | Inactive | Most cysts are not fertile.
Ultrasound features are not pathognomonic. |
| CE5 | Cysts characterised by thick calcified wall which is arch shaped, producing a cone shaped shadow. Degree of calcification varies from partial to complete. | Inactive | Majority of cysts not fertile.
Ultrasound features are not pathognomonic. |
- Pathognomonic features include:
- Unilocular anechoic lesion that are round or oval with a clear, well-defined wall, and souble line sign
- Multivesicular or multiseptated cysts with a wheel-like appearance
- Unilocular cysts with daughter cysts that may have a honeycomb-like appearance
- Cysts with floating laminated membranes with or without daughter cysts
Serology
- Detection of antibodies in serum using indirect hemagglutination, ELISA, or latex agglutination
- Sensitivity 85-98% for liver cysts, 50-60% for lung cysts, and 90-100% for cysts in multiple organs
- Lower sensitivity in early disease (i.e. CE1)
- Cross reacts with other cestodes, including Echinococcus multilocularis, Taenia solium, Fasciola hepatica, filariasis, malignancies, liver cirrhosis, and anti-P1 antibodies
- Positive screening serology should be confirmed with imunoblot assays, IgG subclasses, or arc 5 precipitation testing
- Still cross-reacts with Echinococcus multilocularis and Taenia solium, though less
Management
- The WHO-recommended approach is stage-specific2
- Options include surgery, percutaneous therapy, and medical therapy (with albendazole)
- Surgery is first-line for complicated cysts
- Medical management alone with albendazole is rarely effective
- In resource-poor setting, forgo the albendazole
| Stage | Size | Management |
|---|---|---|
| CE1 | <5 cm | albendazole |
| >5 cm | PAIR + albendazole | |
| CE2 | any | PT + albendazole |
| CE3a | <5 cm | albendazole |
| >5 cm | PAIR + albendazole | |
| CE3b | any | non-PAIR PT + albendazole |
| CE4 | any | watch and wait |
| CE5 | any | watch and wait |
Surgery
- Previously considered the gold standard, though now less invasive options are preferred when possible
- Indicated for:
- Removal of large CE2-CE3b cysts with multiple daughter vesicles
- Single liver cysts, situated superficially, that may rupture spontaneously or as a result of trauma when PTs are not available
- Infected cysts, when PTs are not available
- Cysts communicating with the biliary tree, when PTs are not available
- Cysts exerting pressure on adjacent vital organs
- No specific contraindications
- Must make effort to avoid spilling protoscolices
Percutaneous Treatments
- Treatment targets with the germinal layer or evacuation of the entire endocyst
- Puncture, aspiration, injection, and re-aspiration (PAIR) is the most common
- Injection is with a protoscolicide
- Goal is to kill the germinal layers
- May need continuous catheter drainage for giant (>10 cm) cysts, removed when output <10 mL/day
- Contraindicated with CE2, CE3b, CE4, and CE5, lung cysts, and biliary fistula
- Large-bore catheters + cutting devices + aspiration
- Goal is removal of entire endocyst and daughter cysts from the mother cyst
- Risk of anaphylaxis is quite low, about 1%3
Medication
- May be used in combination with surgerical resection or PAIR, to prevent secondary seeding
- Shouldn't be done too soon before procedure due to potential for becoming more fragile and therefore more likely to rupture
- For simple cysts, usually start the albendazole only 1-3 days before the procedure
- Continued for 4 weeks post-procedurally
- Albendazole 10-15 mg/kg/day in two divided doses, taken with a fat-rich meal
- 30% cure at 3-6 months when medical management alone is used
- Contraindicated with risk of rupture and in early pregnancy, and are not effective with cysts >10 cm
- Monitor for hepatotoxicity, leukopenia, and thrombocytopenia
- Alternatives
- Mebendazole 40-50 mg/kg daily in three divided doses, taken with a fat-rich meal
- Praziquantel 40 mg/kg once weekly, combined with albendazole
Further Reading
- Cystic Echinococcosis. J Clin Microbiol. 2016;54(3):518-523. doi: 10.1128/JCM.02420-15
- Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Tropica. 2010;114:1-16. doi: 10.1016/j.actatropica.2009.11.001
- Treatment guidelines from the WHO's Informal Working Group on Echinococcosis
References
- ^ WHO Informal Working Group. International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. Acta Tropica. 2003;85(2):253-261. doi:10.1016/s0001-706x(02)00223-1.
- ^ Enrico Brunetti, Peter Kern, Dominique Angèle Vuitton. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Tropica. 2010;114(1):1-16. doi:10.1016/j.actatropica.2009.11.001.
- ^ Andreas Neumayr, Giuliana Troia, Chiara de Bernardis, Francesca Tamarozzi, Sam Goblirsch, Luca Piccoli, Christoph Hatz, Carlo Filice, Enrico Brunetti. Simon Brooker. Justified Concern or Exaggerated Fear: The Risk of Anaphylaxis in Percutaneous Treatment of Cystic Echinococcosis—A Systematic Literature Review. PLoS Neglected Tropical Diseases. 2011;5(6):e1154. doi:10.1371/journal.pntd.0001154.