Coccidioides immitis: Difference between revisions
From IDWiki
Coccidioides immitis
Content deleted Content added
No edit summary |
|||
| Line 7: | Line 7: | ||
== Epidemiology == |
== Epidemiology == |
||
[[File:Coccidiomycosis_map.png|thumb|Distribution of coccidiomycosis]] |
|||
* Found in thermic soils in arid regions |
* Found in thermic soils in arid regions |
||
Revision as of 20:34, 13 February 2020
- Dimorphic fungus in patients from arid areas in SW US and Mexico, which is mostly asymptomatic but can cause pulmonary disease, and rarely disseminated disease. Also called Joaquin Valley fever.
Microbiology
- Dimorphic fungus with barrel-shaped arthroconidia in the septated hyphal filamentous form
- Coccidiomycosis also caused by Coccidioides posadasii outside of California
Epidemiology
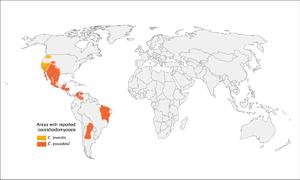
- Found in thermic soils in arid regions
- May have an association with small desert mammals, though of unclear significance
- C. immitis in California, C. posadasii outside of California (southwestern US, Mexico, Central and South America)
- Incidence in US is increasing over the past few decades, including emergence in Washington state
- Unlikely to be from increased detection
- 95% of cases are in California and Arizona
- Exposures include archaeological sites, desert exposures, dust storms, prisons
- It is a significant laboratory hazard
- There is a strain with a mutation that can cause aggressive invasive disease in younger adults
Pathophysiology
- Arthroconidia break off of the filamentous form and are inhaled (or inoculated)
- The then transform into invasive yeast form, characterized by spherules that contain hundreds of endospores
- The spherules rupture, releasing the endospores and continuing the cycle
Clinical Presentation
- Variable, from asymptomatic or mild (60%), to symptomatic (40%), to disseminated and fulminant
- Incubation period 1-3 weeks
- Common symptoms:
- General: fever, arthralgia, myalgia, night sweats
- Pulmonary: cough, chest pain
- May be a common cause of community-acquired pneumonia in some endemic regions.
- Non-specific maculopapular rash (days to weeks after initial fever)
- May have peripheral eosinophilia
- Pulmonary infection may present as an acute pneumonia to a chronic pneumonia with fibronodular or fibrocavitary appearance
- May also have a solitary nodule or a pleural effusion
- Skin infection can present at site of inoculation, or disseminated
- Can cause verrucous granulomatous lesions
- Erythema nodosum may be seen
- Erythema multiforme
- Non-specific rash
- Osteomyelitis and native joint infections, with the vertebral being to the most common site of infection
- May also cause arthralgias
- CNS infection with meningitis, presenting as a chronic headache weeks to months after initial infection
- Onset of meningitis is usually around 5 weeks following onset of respiratory symptoms
- Typically basilar meningitis but can be other
- Can cause prostatitis
- Symptoms may take weeks to many months to resolve
Desert rheumatism
- Erythema nodosum
- Fevers
- Arthralgias
Complications
- Chronic pulmonary disease:
- Residual lung nodules (~5%)
- Lung cavity (~5%)
- Extra-pulmonary dissemination (skin, bones, joints, CNS): ≤1%
Risk factors for disseminated disease
- Immunosuppression, including HIV/AIDS, pregnancy, TNF inhibitors, corticosteroids, and transplantation
- More common in non-Caucasian populations
- Age >65 years, diabetes, underlying cardiac or respiratory disease
- High complement fixation titre >1:16
Differential Diagnosis
- Infectious
- Bacterial: routine bacterial infections
- Fungal: cryptococcosis, other endemic fungi
- Mycobacterial: TB
- Viral?
- Non-infectious
Diagnosis
- Direct microscopy: typical spherules
- Culture: fluffy white colonies on routine bacterial or fungal cultures, often within a few days (grows quickly)
- Serology
- Immunodiffusion (ID) for IgM and IgG
- IgM on CSF for coccioidal meningitis
- Complement fixation for IgG
- Beta glucan in blood or CSF, but isn't specific
- Coccidioidal antigen (urine and serum), only useful in disseminated disease
- Immunodiffusion (ID) for IgM and IgG
- For CNS involvement:
- Check opening pressure, send for fungal culture (25% sensitive), CSF serology (ID or CF, 30-60% sensitive), CSF coccidioidal antigen
- Glucose normal or low, protein high or normal, predominately lymphocytosis, may have eosinophils
- Rule out Cryptococcus and TB
Management
- Many do not need treatment, as infection is asymptomatic or self-limited
- Indications
- High-risk groups: immunocompromised/suppressed, diabetes, elderly, comorbidities
- High-risk infection: extensive pulmonary disease, severe symptoms, persistent disease (especially lasting more than 2 months), complement fixation >1:16
- Extrapulmonary disease
- Treatment regimens
- Fluconazole 400-800 mg/day
- May use itraconazole, voriconazole, posaconazole, or isavuconazole
- May use amphotericin if life-threatening infections, pregnancy, or refractory to azole therapy
- Slight preference for itra for bone & joint infections
- Duration
- Acute pulmonary disease: 3 to 6 months, or longer
- Chronic pulmonary infection: 8 to 12 months, or longer
- Bone and joint disease: ampho B induction x 3 months followed by 3+ years of azoles
- Meningitis
- High-dose fluconazole until clinical improvement, then lifelong fluconazole 400 mg daily
- Daily therapeutic LP titrated to opening pressure (like crypto)
Laboratory Exposure
- Evacuate lab, seal lab, call Biosafety Officer on call
- Assess risk to each individual exposed
- Lower early in culture when it's yeast-like, higher later when it's a well-grown filamentous fungus (usually by 7 to 10 days)
- Lifting the lid once likely less than breaking the container
- However, note that in lab exposures, the number of arthroconidia inhaled is often much higher than a natural inoculum, and attack rates are higher than natural exposure
- For exposed personnel
- Get baseline serology (if prior exposure, they're at lower risk)
- Treat with therapeutic-dose itra or fluc 400 mg daily for 6 weeks, as prophylaxis
- If pregnant, would have to consider prophylactic amphotericin; use of fluconazole may depend on trimester
- Repeat serology after prophylaxis; if seroconversion, then consider treating for another few months
- If they become symptomatic during or after prophylaxis, either with fever or respiratory symptoms, they should be further evaluated
- Serology can lag by 3 to 12 weeks following symptoms
- Continue to follow for up to 1 year
References
- ^ Cecilia M. Jude, Nita B. Nayak, Maitraya K. Patel, Monica Deshmukh, Poonam Batra. Pulmonary Coccidioidomycosis: Pictorial Review of Chest Radiographic and CT Findings. RadioGraphics. 2014;34(4):912-925. doi:10.1148/rg.344130134.
- ^ Ian H. McHardy, Bridget Barker, George R. Thompson. Romney M. Humphries. Review of Clinical and Laboratory Diagnostics for Coccidioidomycosis. Journal of Clinical Microbiology. 2023;61(5). doi:10.1128/jcm.01581-22.