Helicobacter pylori: Difference between revisions
From IDWiki
Helicobacter pylori
Content deleted Content added
No edit summary |
mNo edit summary |
||
| (5 intermediate revisions by the same user not shown) | |||
| Line 3: | Line 3: | ||
*Slow-growing [[Stain::Gram-negative]] microaerophilic [[Shape::bacillus]] with a curve, gull-wing, or spiral appearance |
*Slow-growing [[Stain::Gram-negative]] microaerophilic [[Shape::bacillus]] with a curve, gull-wing, or spiral appearance |
||
*Oxidase-[[Oxidase::positive]] and urease-[[Urease::positive]] |
*Oxidase-[[Oxidase::positive]] and urease-[[Urease::positive]] |
||
*Major cause of peptic ulcer disease and gastric cancer |
*Major cause of peptic ulcer disease and gastric cancer worldwide |
||
=== Pathophysiology === |
|||
*Urease neutrolizes acid and induces angiogenesis |
|||
*Strains with CagA, VacA, and BabA are associated with more cellular metaplasia |
|||
=== Epidemiology === |
|||
* Present worldwide |
|||
* About half of the world's population is estimated to have chronic infection[[CiteRef::zamani2018sy]] |
|||
* Usually acquired during infancy or childhood |
|||
* Transmission is likely fecal-oral or oral-oral |
|||
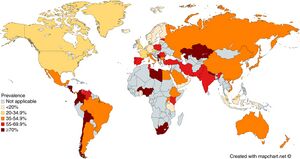
[[File:Prevalence of ''Helicobacter pylori'' infection across the world.jpg|thumb|Prevalence of Helicobacter pylori infection across the world. From: Zamani ''et al''. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. ''Aliment Pharmacol Ther''. 2018;47(7):868-876. doi: [https://doi.org/10.1111/apt.14561 10.1111/apt.14561].]] |
|||
== Clinical Manifestations == |
|||
* Mostly asymptomatic |
|||
* Complications include: |
|||
** Peptic ulcer disease in 1 to 10% |
|||
** Gastric cancer in 0.1 to 3% |
|||
** MALT lymphoma in 0.01% |
|||
== Diagnosis == |
|||
* Gastroscopy with biopsy for histopathology is the gold standard |
|||
* Culture is challenging but necessary for phenotyping susceptibility testing |
|||
=== Urea Breath Test === |
|||
* Patient is fed urea labelled with <sup>13</sup>C or <sup>14</sup>C isotopes, which is hydrolyzed into ammonia and isotope-labelled CO<sub>2</sub>, which is detected in exhaled breath 30 minutes later and measured by mass spectrometry (or other method) |
|||
** The delta over baseline (DOB) (i.e. increase in labelled CO<sub>2</sub>) is compared to a threshold |
|||
** Cutoff DOB is usually 5% |
|||
* False negatives may be seen with PPIs (which should be held for 7 days before test), recent antibiotics (should be off of them for 4 weeks before test), bleeding ulcers (should be resolved before test), and corpus-predominant gastritis |
|||
* False positives may be seen with [[Helicobacter heilmannii]] and rarely with other [[urease-producing organisms]] such as [[Proteus mirabilis]], [[Citrobacter freundii]], [[Klebsiella pneumoniae]], [[Enterobacter cloacae]], [[Staphylococcus aureus]], [[Staphylococcus capitis]] subsp. ''urealyticus'' |
|||
=== Stool Antigen Test === |
|||
* Non-invasive testing, and preferred to pediatric patients |
|||
* Based on ELISA, immunochromatographic assay, and CLIA |
|||
* Affected by PPIs (should be held for 7-14 days)[[CiteRef::manes2001ac]], antibiotics, bismuth-containing medications, and [[N-acetylcysteine]] |
|||
* Sample is temperature sensitive: max 24 hours at room temperature, 72 hours at 4ºC, or long-term if frozen |
|||
=== Serology === |
|||
* Includes IgM, IgA, and IgG antibodies |
|||
* More false positives with IgA and IgM |
|||
* Post-treatment IgG titres can take 6-12 months to fall below 50% compared to pre-treatment |
|||
* Not affected by concurrent medications, unlike other non-invasive tests |
|||
* Accuracy varies by strain, so ideally should use locally-validated tests |
|||
=== Test of Cure === |
|||
* Urea breath test is preferred to stool antigen |
|||
* Serology not helpful |
|||
==Management== |
==Management== |
||
| Line 9: | Line 63: | ||
*Treatment is with combination therapy for 14 days followed by confirmation of eradication |
*Treatment is with combination therapy for 14 days followed by confirmation of eradication |
||
*First-line: |
*First-line: |
||
**PBMT ( |
**[[PBMT]] (BMT Quad): bismuth subsalicylate 524 mg p.o. four time daily, metronidazole 500 mg p.o. three to four times daily, tetracycline 500 mg p.o. four times daily for 14 days |
||
**PAMC (PPI, [[amoxicillin]], |
**[[PAMC]] (CLAMET Quad): PPI twice daily, [[amoxicillin]] 1 g p.o. twice daily, metronidazole 500 mg p.o. twice daily, and [[clarithromycin]] 500 mg p.o. twice daily for 14 days |
||
**PAC (PPI, [[amoxicillin]], [[clarithromycin]]), PMC (PPI, [[metronidazole]], [[clarithromycin]]), or PAM (PPI, [[amoxicillin]], [[metronidazole]]) only in areas with clarithromycin resistance <15% or with proven high local eradication rates >85% |
**[[PAC]] (PPI, [[amoxicillin]], [[clarithromycin]]), PMC (PPI, [[metronidazole]], [[clarithromycin]]), or [[PAM]] (PPI, [[amoxicillin]], [[metronidazole]]) only in areas with [[clarithromycin]] resistance <15% or with proven high local eradication rates >85% |
||
*Prior treatment failure: |
*Prior treatment failure: |
||
**PBMT |
**[[PBMT]]: PPI twice daily, bismuth subsalicylate 524 mg p.o. four times daily, [[metronidazole]] 500 mg p.o. three to four times daily, [[tetracycline]] 500 mg p.o. four times daily |
||
**[[PAL]]: PPI twice daily, [[levofloxacin]] 500 mg p.o. once daily, and [[amoxicillin]] 750 mg p.o. three times daily for 14 days |
|||
**PAL (PPI, [[amoxicillin]], [[levofloxacin]]) |
|||
**PAR |
**[[PAR]]: PPI twice daily, amoxicillin 750 mg p.o. three times daily, and rifabutin 300 mg p.o. once daily for 10-14 days |
||
| ⚫ | |||
*Doses: |
|||
**PBMT |
|||
***Bismuth subsalicylate 524 mg (2x 262 mg tablets) PO qid |
|||
***[[Metronidazole]] 500 MG PO tid or qid |
|||
***PPI: esomeprazole 20 mg, lansoprazole 30 mg, omeprazole 20 mg, pantoprazole 40 mg, or rabeprazole 20 mg |
|||
****Some areas use double dosing |
|||
***Tetracycline 500 mg PO qid |
|||
**Others |
|||
***Amoxicillin 1000 mg PO bid |
|||
***Clarithromycin 500 mg PO bid |
|||
***Levofloxacin 500 mg PO daily |
|||
***Metronidazole 500 mg PO bid |
|||
***Rifabutin 150 mg PO bid |
|||
***PPI as above |
|||
| ⚫ | |||
*Confirmation of eradication should be done 4 weeks following treatment |
*Confirmation of eradication should be done 4 weeks following treatment |
||
*Recommended order of treatment, if persistently positive: |
|||
**[[PBMT]] (or [[PAMC]]) |
|||
**[[PAMC]] (or [[PBMT]]) |
|||
**[[PAL]] |
|||
**[[PAR]] vs. repeat endoscopy for culture and susceptibility testing |
|||
=== Antibiotic Resistance === |
|||
* Mechanisms: |
|||
** [[Amoxicillin]] resistance is caused by modified PBPs (rather than [[β-lactamases]]) |
|||
** [[Clarithromycin]] resistance is caused by point mutations in the 23S rRNA of 50S ribosomal subunit |
|||
** [[Metronidazole]] resistance is caused by mutations in RdxA and FrxA enzymes |
|||
** [[Levofloxacin]] resistance is caused by point mutations in DNA gyrase (''gyrA'' or ''gyrB'') |
|||
** [[Tetracycline]] resistance is uncommon and not fully understood |
|||
** [[Rifabutin]] resistance is uncommon and caused by mutations in DNA-dependent RNA polymerase |
|||
* The most important regional rates of resistance to pay attention to when choosing empiric treatment is to [[clarithromycin]] and [[metronidazole]], since they are most frequent |
|||
== |
==Further Reading== |
||
* |
*''H. pylori'' Enhanced Primary Care Pathway: [[2016 version]], [https://divisionsbc.ca/sites/default/files/inline-files/HPYLORI%20Enhanced%20Primary%20Care%20Pathway%202019_0.pdf 2019 version], [https://www.specialistlink.ca/files/HPylori_PCPathway_April112020.pdf 2020 version] |
||
* |
*The Toronto Consensus for the Treatment of ''Helicobacter pylori'' Infection in Adults. ''Gastroenterol''. 2016;151:51–69. doi: [https://doi.org/10.1053/j.gastro.2016.04.006 10.1053/j.gastro.2016.04.006] |
||
* |
*Houston Consensus Conference on Testing for ''Helicobacter pylori'' Infection in the United States. ''Clin Gastroenterol Hepatol''. 2018;16(7):992-1002.e6. doi: [https://doi.org/10.1016/j.cgh.2018.03.013 10.1016/j.cgh.2018.03.013] |
||
{{DISPLAYTITLE:''Helicobacter pylori''}} |
{{DISPLAYTITLE:''Helicobacter pylori''}} |
||
[[Category:Gram-negative bacilli]] |
[[Category:Gram-negative bacilli]] |
||
Latest revision as of 18:12, 19 September 2024
Background
- Slow-growing Gram-negative microaerophilic bacillus with a curve, gull-wing, or spiral appearance
- Oxidase-positive and urease-positive
- Major cause of peptic ulcer disease and gastric cancer worldwide
Pathophysiology
- Urease neutrolizes acid and induces angiogenesis
- Strains with CagA, VacA, and BabA are associated with more cellular metaplasia
Epidemiology
- Present worldwide
- About half of the world's population is estimated to have chronic infection1
- Usually acquired during infancy or childhood
- Transmission is likely fecal-oral or oral-oral
Clinical Manifestations
- Mostly asymptomatic
- Complications include:
- Peptic ulcer disease in 1 to 10%
- Gastric cancer in 0.1 to 3%
- MALT lymphoma in 0.01%
Diagnosis
- Gastroscopy with biopsy for histopathology is the gold standard
- Culture is challenging but necessary for phenotyping susceptibility testing
Urea Breath Test
- Patient is fed urea labelled with 13C or 14C isotopes, which is hydrolyzed into ammonia and isotope-labelled CO2, which is detected in exhaled breath 30 minutes later and measured by mass spectrometry (or other method)
- The delta over baseline (DOB) (i.e. increase in labelled CO2) is compared to a threshold
- Cutoff DOB is usually 5%
- False negatives may be seen with PPIs (which should be held for 7 days before test), recent antibiotics (should be off of them for 4 weeks before test), bleeding ulcers (should be resolved before test), and corpus-predominant gastritis
- False positives may be seen with Helicobacter heilmannii and rarely with other urease-producing organisms such as Proteus mirabilis, Citrobacter freundii, Klebsiella pneumoniae, Enterobacter cloacae, Staphylococcus aureus, Staphylococcus capitis subsp. urealyticus
Stool Antigen Test
- Non-invasive testing, and preferred to pediatric patients
- Based on ELISA, immunochromatographic assay, and CLIA
- Affected by PPIs (should be held for 7-14 days)2, antibiotics, bismuth-containing medications, and N-acetylcysteine
- Sample is temperature sensitive: max 24 hours at room temperature, 72 hours at 4ºC, or long-term if frozen
Serology
- Includes IgM, IgA, and IgG antibodies
- More false positives with IgA and IgM
- Post-treatment IgG titres can take 6-12 months to fall below 50% compared to pre-treatment
- Not affected by concurrent medications, unlike other non-invasive tests
- Accuracy varies by strain, so ideally should use locally-validated tests
Test of Cure
- Urea breath test is preferred to stool antigen
- Serology not helpful
Management
- Treatment is with combination therapy for 14 days followed by confirmation of eradication
- First-line:
- PBMT (BMT Quad): bismuth subsalicylate 524 mg p.o. four time daily, metronidazole 500 mg p.o. three to four times daily, tetracycline 500 mg p.o. four times daily for 14 days
- PAMC (CLAMET Quad): PPI twice daily, amoxicillin 1 g p.o. twice daily, metronidazole 500 mg p.o. twice daily, and clarithromycin 500 mg p.o. twice daily for 14 days
- PAC (PPI, amoxicillin, clarithromycin), PMC (PPI, metronidazole, clarithromycin), or PAM (PPI, amoxicillin, metronidazole) only in areas with clarithromycin resistance <15% or with proven high local eradication rates >85%
- Prior treatment failure:
- PBMT: PPI twice daily, bismuth subsalicylate 524 mg p.o. four times daily, metronidazole 500 mg p.o. three to four times daily, tetracycline 500 mg p.o. four times daily
- PAL: PPI twice daily, levofloxacin 500 mg p.o. once daily, and amoxicillin 750 mg p.o. three times daily for 14 days
- PAR: PPI twice daily, amoxicillin 750 mg p.o. three times daily, and rifabutin 300 mg p.o. once daily for 10-14 days
- Duration generally 14 days
- Confirmation of eradication should be done 4 weeks following treatment
- Recommended order of treatment, if persistently positive:
Antibiotic Resistance
- Mechanisms:
- Amoxicillin resistance is caused by modified PBPs (rather than β-lactamases)
- Clarithromycin resistance is caused by point mutations in the 23S rRNA of 50S ribosomal subunit
- Metronidazole resistance is caused by mutations in RdxA and FrxA enzymes
- Levofloxacin resistance is caused by point mutations in DNA gyrase (gyrA or gyrB)
- Tetracycline resistance is uncommon and not fully understood
- Rifabutin resistance is uncommon and caused by mutations in DNA-dependent RNA polymerase
- The most important regional rates of resistance to pay attention to when choosing empiric treatment is to clarithromycin and metronidazole, since they are most frequent
Further Reading
- H. pylori Enhanced Primary Care Pathway: 2016 version, 2019 version, 2020 version
- The Toronto Consensus for the Treatment of Helicobacter pylori Infection in Adults. Gastroenterol. 2016;151:51–69. doi: 10.1053/j.gastro.2016.04.006
- Houston Consensus Conference on Testing for Helicobacter pylori Infection in the United States. Clin Gastroenterol Hepatol. 2018;16(7):992-1002.e6. doi: 10.1016/j.cgh.2018.03.013
References
- ^ M. Zamani, F. Ebrahimtabar, V. Zamani, W. H. Miller, R. Alizadeh‐Navaei, J. Shokri‐Shirvani, M. H. Derakhshan. Systematic review with meta‐analysis: the worldwide prevalence of Helicobacter pylori infection. Alimentary Pharmacology & Therapeutics. 2018;47(7):868-876. doi:10.1111/apt.14561.
- ^ G. Manes, A. Balzano, G. Iaquinto, C. Ricci, M. M. Piccirillo, N. Giardullo, A. Todisco, M. Lioniello, D. Vaira. Accuracy of the stool antigen test in the diagnosis of Helicobacter pylori infection before treatment and in patients on omeprazole therapy. Alimentary Pharmacology & Therapeutics. 2001;15(1):73-79. doi:10.1046/j.1365-2036.2001.00907.x.