Aminoglycosides: Difference between revisions
From IDWiki
Content deleted Content added
No edit summary |
|||
| (5 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
<br /> |
|||
==Background== |
==Background== |
||
*Derived from [[Streptomyces |
*Derived from [[Streptomyces]] (mycins & kacins) or [[Micromonospora]] (micins) |
||
===Mechanism of Action=== |
===Mechanism of Action=== |
||
| Line 14: | Line 12: | ||
*Good coverage of Gram-negative aerobes |
*Good coverage of Gram-negative aerobes |
||
**Except Stenotrophomonas and Burkholderia |
**Except [[Stenotrophomonas]] and [[Burkholderia]] |
||
*Streptomycin also covers mycobacterium |
*[[Streptomycin]] also covers mycobacterium |
||
*Some protozoal coverage |
*Some protozoal coverage |
||
*Can cover Gram-positives if cell wall is disrupted (e.g. by beta-lactam) |
*Can cover Gram-positives if cell wall is disrupted (e.g. by beta-lactam) |
||
| Line 22: | Line 20: | ||
*Altered 50S ribosomal subunit |
*Altered 50S ribosomal subunit |
||
*Decreased uptake and accumulation (Pseudomonas) |
*Decreased uptake and accumulation ([[Pseudomonas]]) |
||
*Decreased membrane permeability |
*Decreased membrane permeability |
||
*Efflux ( |
*Efflux pump ([[Escherichia coli]]) |
||
*Aminoglycoside-modifying enzymes (Enterococcus) |
*Aminoglycoside-modifying enzymes ([[Enterococcus]]) |
||
===Pharmacokinetics and Pharmacodynamics=== |
===Pharmacokinetics and Pharmacodynamics=== |
||
| Line 38: | Line 36: | ||
===Initial Dose=== |
===Initial Dose=== |
||
If actual body weight more than 20% higher than ideal body weight, need to calculate adjusted body weight (ABW) |
*If actual body weight more than 20% higher than [https://www.mdcalc.com/ideal-body-weight-adjusted-body-weight ideal body weight], need to calculate [https://www.mdcalc.com/ideal-body-weight-adjusted-body-weight adjusted body weight] (ABW) |
||
$$ABW = IBW + 0.4 \times (actual BW - IBW)$$ |
$$ABW = IBW + 0.4 \times (actual BW - IBW)$$ |
||
| Line 44: | Line 42: | ||
===Traditional Dosing=== |
===Traditional Dosing=== |
||
*Q8H dosing |
|||
*Used for Enterococcus IE, meningitis, septic shock, ascites, AKI/CKD, pregnancy, surgical prophylaxis, burns, osteomyelitis |
*Used for [[Enterococcus]] IE, [[meningitis]], [[septic shock]], [[ascites]], [[AKI]]/[[CKD]], [[pregnancy]], surgical prophylaxis, [[Burn infection|burns]], [[osteomyelitis]] |
||
*1. |
*1.7 mg/kg (5-7.5 mg/kg [[amikacin]]) IV q8h |
||
===Extended Interval Dosing=== |
===Extended Interval Dosing=== |
||
*Q24H dosing, which is safer but less well-studied |
|||
*7mg/kg (15mg/kg amikacin) |
|||
*7 mg/kg (15 mg/kg [[amikacin]]) IV, frequency depends on [[CrCl]] |
|||
| ⚫ | |||
*CrCl ≥60 q24h |
**[[CrCl]] ≥60 q24h |
||
**[https://www.mdcalc.com/creatinine-clearance-cockcroft-gault-equation CrCl] 40-59 q36h |
|||
*CrCl 40-59 q36h |
|||
**[https://www.mdcalc.com/creatinine-clearance-cockcroft-gault-equation CrCl] 20-39 q48h |
|||
*CrCl 20-39 q48h |
|||
*CrCl ≤19 don't use |
**[https://www.mdcalc.com/creatinine-clearance-cockcroft-gault-equation CrCl] ≤19 don't use |
||
| ⚫ | |||
===Dialysis Dosing=== |
===Dialysis Dosing=== |
||
| Line 62: | Line 62: | ||
===Synergy=== |
===Synergy=== |
||
* |
*1 mg/kg divided q8-12h, peak target 3-5, trough <2 |
||
===Monitoring=== |
===Monitoring=== |
||
| Line 68: | Line 68: | ||
====Peak==== |
====Peak==== |
||
* |
*30 minutes after third dose |
||
*Response is based on peak:MIC ratio, target is 8-10 times |
*Response is based on peak:MIC ratio, target is 8-10 times |
||
*If below target, increase dose |
*If below target, increase dose |
||
| Line 74: | Line 74: | ||
====Trough==== |
====Trough==== |
||
*Prior to 4th dose, or a random level at 24 |
*Prior to 4th dose, or a random level at 24 to 48h in renal failure |
||
*Side effects are predicted by trough levels |
*Side effects are predicted by trough levels |
||
* |
*[[Tobramycin]] <0.5 (extended) or <2 (traditional) |
||
*Amikacin <1 (extended) or <?? (traditional) |
*[[Amikacin]] <1 (extended) or <?? (traditional) |
||
*If above target, increase interval |
*If above target, increase interval |
||
| Line 84: | Line 84: | ||
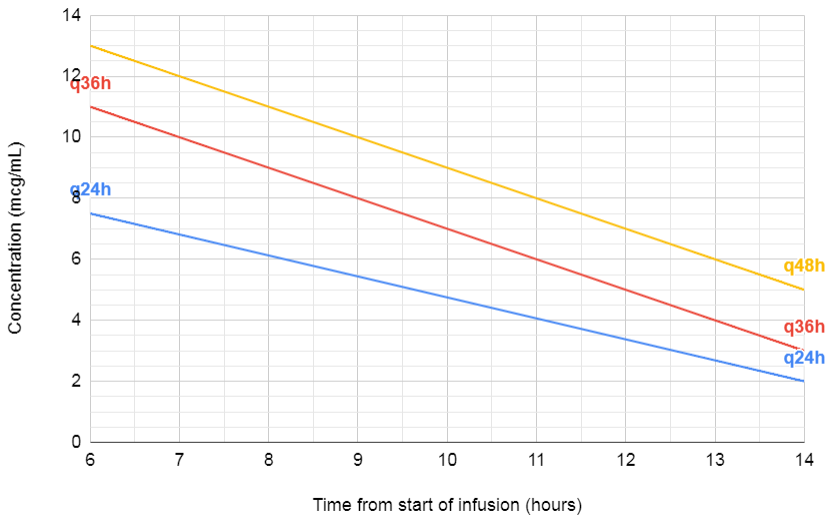
[[File:Hartford_nomogram.png]] |
[[File:Hartford_nomogram.png]] |
||
*Dosing interval is whichever is the line just above the random level |
|||
*Double the concentration for [[amikacin]] |
*Double the concentration for [[amikacin]] |
||
Latest revision as of 18:46, 11 January 2024
Background
- Derived from Streptomyces (mycins & kacins) or Micromonospora (micins)
Mechanism of Action
- Requires electron transport chain (ETC) to cross over the membrane
- Anaerobes are therefore inherently resistant
- Reversibly binds 30S ribosomal subunit, which stops proofreading and causes accumulation of bad proteins
Spectrum of Activity
- Good coverage of Gram-negative aerobes
- Except Stenotrophomonas and Burkholderia
- Streptomycin also covers mycobacterium
- Some protozoal coverage
- Can cover Gram-positives if cell wall is disrupted (e.g. by beta-lactam)
Mechanisms of Resistance
- Altered 50S ribosomal subunit
- Decreased uptake and accumulation (Pseudomonas)
- Decreased membrane permeability
- Efflux pump (Escherichia coli)
- Aminoglycoside-modifying enzymes (Enterococcus)
Pharmacokinetics and Pharmacodynamics
- Poor membrane penetration, therefore doesn't cross over into lungs and CSF
- Half-life 2-3 hours (longer in CKD)
- Excreted 99% unchanged in urine
- Displays concentration-depedent killing with a prolonged post-antibiotic effect (2-13 hours)
Dosing
Initial Dose
- If actual body weight more than 20% higher than ideal body weight, need to calculate adjusted body weight (ABW)
$$ABW = IBW + 0.4 \times (actual BW - IBW)$$
Traditional Dosing
- Q8H dosing
- Used for Enterococcus IE, meningitis, septic shock, ascites, AKI/CKD, pregnancy, surgical prophylaxis, burns, osteomyelitis
- 1.7 mg/kg (5-7.5 mg/kg amikacin) IV q8h
Extended Interval Dosing
- Q24H dosing, which is safer but less well-studied
- 7 mg/kg (15 mg/kg amikacin) IV, frequency depends on CrCl
- Use Hartford nomogram with a random level (but remember to halve the amikacin level first)
Dialysis Dosing
- Pre-HD levels with post-HD doses, though this may change
Synergy
- 1 mg/kg divided q8-12h, peak target 3-5, trough <2
Monitoring
Peak
- 30 minutes after third dose
- Response is based on peak:MIC ratio, target is 8-10 times
- If below target, increase dose
Trough
- Prior to 4th dose, or a random level at 24 to 48h in renal failure
- Side effects are predicted by trough levels
- Tobramycin <0.5 (extended) or <2 (traditional)
- Amikacin <1 (extended) or <?? (traditional)
- If above target, increase interval
Hartford Nomogram
- Dosing interval is whichever is the line just above the random level
- Double the concentration for amikacin
Safety
Adverse Drug Reactions
- Nephrotoxicity (0-50%), usually non-oliguric AKI with decreased Ca/Mg resorption, often reversible
- Decreased protein synthesis
- Decreased cellular respiration
- Increased apoptosis
- Necrosis in proximal tubules
- Ototoxicity (0-60%), irreversible
- Cumulative effect
- Distribute into the perilymph of the ear, and cause free radical formation causing apoptosis of hair cells
- Needs hearing tests, because it can be subclinical
- Monitor audiometry weekly
- Vestibulotoxicity (0-20%), irreversible
- Rarely, neuromuscular blockade
Monitoring
- Trough levels
- Creatinine
- Weekly audiometry